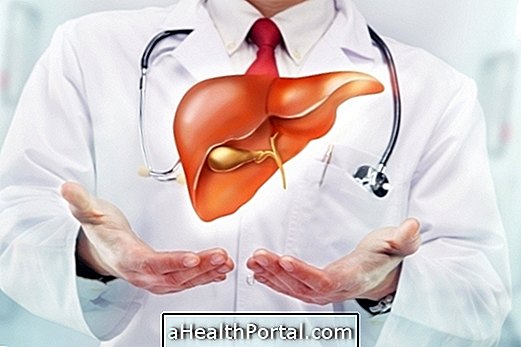
Ocaliva is the brand name of an obeticolic acid-based remedy, which acts in the liver to suppress bile acid production, being a new treatment option against primary biliary cholangitis, a disease that had only one form of treatment, the use of ursodeoxycholic acid which was not effective in almost half of the patients.
This new drug is manufactured by pharmaceutical Intercept and despite having been approved by the FDA has not yet been approved by Anvisa and therefore is not marketed in Brazil, pending authorization.

Indications
Ocaliva is indicated for the treatment of primary biliary cholangitis in combination with ursodeoxycholic acid and is useful for adults who did not respond well to treatment with ursodeoxycholic acid alone for 1 year of treatment.
How to use
The starting dose of Ocaliva is 1 5 mg tablet once daily for 3 months. After this period the test should be performed again to evaluate the ALP and bilirubin and if the reduction of these values is not adequate and the person tolerates the drug well without intense itching by the body, the doctor can increase the dose to 2 tablets of 5 mg once daily.
If itching is severe what can be done is to maintain the dose of 5 mg daily or stop using the medicine for 2 weeks and then restart the intake, noting if there is a decrease in this side effect.
Side effects
There may be symptoms such as itching through the body, fatigue, pain and discomfort, redness of the skin, sore throat, joint pain, thyroid disorders, heart palpitations, constipation and eczema.
Contraindications
Ocaliva is contraindicated for people with biliary obstruction and is not advised for people taking Varfina. Its use in pregnancy and during breastfeeding should only be done with guidance from the obstetrician and if the benefit of the use of the medicine is greater than its risk. This medicine is not recommended for children.





.jpg)